Standard enthalpy change of combustion
The standard enthalpy of combustion is the enthalpy change when one mole of a substance completely reacts with oxygen under standard thermodynamic conditions (although experimental values are usually obtained under different conditions and subsequently adjusted). By definition, combustion reactions are always exothermic and so enthalpies of combustion are always negative, although the values for individual combustions may vary.
The most common way of calculating the enthalpy change of combustion (or formation) is by using a Hess cycle or by using numerical based bond enthalpies. It is commonly denoted as 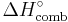 or
or 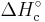 . When the enthalpy required is not a combustion, it can be denoted as
. When the enthalpy required is not a combustion, it can be denoted as 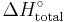 . Enthalpies of combustion are typically measured using bomb calorimetry, and have units of energy (typically kJ); strictly speaking, the enthalpy change per mole of substance combusted is the standard molar enthalpy of combustion (which typically would have units of kJ mol−1).
. Enthalpies of combustion are typically measured using bomb calorimetry, and have units of energy (typically kJ); strictly speaking, the enthalpy change per mole of substance combusted is the standard molar enthalpy of combustion (which typically would have units of kJ mol−1).